Dextromethorphan HBR
Dextromethorphan HBR Specification
- Dosage Form
- Tablet, syrup, powder (bulk raw material)
- Origin of Medicine
- Synthetic
- Salt Composition
- Dextromethorphan Hydrobromide
- Indication
- Dry cough, non-productive cough
- Pacakaging (Quantity Per Box)
- As per customer requirement
- Drug Type
- API (Active Pharmaceutical Ingredient)
- Ingredients
- Dextromethorphan Hydrobromide
- Physical Form
- Powder
- Function
- Antitussive (Cough Suppressant)
- Recommended For
- Cough Suppression
- Dosage
- As prescribed by physician
- Dosage Guidelines
- Take orally with or without food as directed by physician
- Suitable For
- Adults and Children
- Quantity
- Varies as per requirement
- Storage Instructions
- Store in cool, dry place away from direct sunlight
Dextromethorphan HBR Trade Information
- Minimum Order Quantity
- 5000 Pieces
- Supply Ability
- 3000 Pieces Per Month
- Delivery Time
- 1 Days
- Main Domestic Market
- All India
About Dextromethorphan HBR
We are an ISO 9002-2008certified company engaged in manufacturing & marketing of Pharmaceutical Formulations majority of these are Non DPCO Products, and newDCGI approved molecules e.g. Tablets, Capsules, Liquids, Dry Syrups, Nutraceutical, Herbal, various advanced specialty Injectable products and World Class Packing's(ALU-ALU)which interest Medical Fraternity.
We have in our portfolio, a wide range of products, either different in formulations or having advance technology e.g.Rapid Release (RR) technology or having certain U.S Ps. (Unique selling propositions) which keeps our brand above the mushrooms of many "ME Too's" in the market.
Required interested buyers for Veterinary monopoly rights/PCD offers In Tripura States
Udaipur | Agartala | Ambasa |
Kailashahar | Kolasib | Belonia |
Salema | Narayanpur | Khowai |
High-Quality Antitussive API
Our Dextromethorphan HBR is produced to stringent pharmaceutical standards, ensuring a minimum of 99% purity by HPLC. The API is suitable for formulation in a variety of cough suppressing dosage forms, including syrups, tablets, and lozenges. This synthetic compound is designed for reliability and efficacy, making it an essential ingredient in pharmaceutical manufacturing.
Safe and Versatile Formulation
With its solubility in water and ethanol and a pH of 5.07.0 for a 1% solution, Dextromethorphan HBR is easy to incorporate into various product formulations. Its stability and 3-year shelf life allow for flexible storage and handling. Supplied in double-bagged HDPE drums or as per specific requirements, it ensures product safety and integrity during distribution.
Therapeutic Use and Benefits
Dextromethorphan Hydrobromide serves as an effective suppressant for dry and non-productive coughs. It functions by acting on the cough center in the brain, providing relief from persistent coughing without causing drowsiness. This makes it a preferred choice for manufacturers of over-the-counter and prescription cough treatments offered to both children and adults.
FAQs of Dextromethorphan HBR:
Q: How is Dextromethorphan HBR typically used in pharmaceutical manufacturing?
A: Dextromethorphan HBR is most commonly utilized in the production of cough syrups, lozenges, and tablets. As an active pharmaceutical ingredient, it is integrated into formulations designed to suppress dry, non-productive coughs.Q: What are the recommended storage conditions for Dextromethorphan HBR?
A: Dextromethorphan HBR should be stored in a cool, dry environment, away from direct sunlight, to maintain its stability and effectiveness. Proper storage ensures the products quality over its 3-year shelf life.Q: When should Dextromethorphan HBR-based products be taken?
A: Products containing Dextromethorphan HBR are typically taken as soon as symptoms of a dry or non-productive cough appear. Dosage and timing should follow a physicians prescription and specific product guidelines.Q: Where can Dextromethorphan HBR be purchased or sourced?
A: You can obtain pharmaceutical-grade Dextromethorphan HBR from approved distributors, manufacturers, suppliers, or traders in India, packaged according to customer requirements to ensure safety and compliance.Q: What makes Dextromethorphan HBR beneficial as an antitussive agent?
A: Dextromethorphan HBR provides reliable cough suppression without causing drowsiness, making it suitable for both adults and children. Its high purity and efficacy enhance its effectiveness in treating dry cough.Q: How should Dextromethorphan HBR be administered?
A: Dextromethorphan HBR should be administered orally, either with or without food, strictly as prescribed by a healthcare professional. Dosage varies based on age, formulation, and medical need.Q: What is the process of packaging Dextromethorphan HBR?
A: This API is packed in HDPE drums with double polyethylene bags to prevent contamination, or in other packaging as specified by the customer, ensuring secure transport and long-term stability.
Price 25000 INR/ Piece
- Minimum Order Quantity
- 5000 Pieces
- Supply Ability
- 3000 Pieces Per Month
- Delivery Time
- 1 Days
- Main Domestic Market
- All India

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Tablets Category
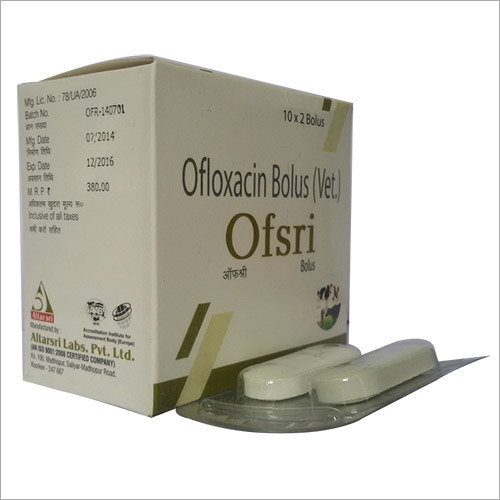
Ofloxacin Bolus Tablets
Price 25150 INR / Piece
Minimum Order Quantity : 5000 Pieces
Physical Form : Tablets
Drug Type : Specific Drug
Recommended For : Cattle, Buffalo, Livestock
Storage Instructions : Store in a cool, dry place, protected from light
Cloxacillin Tablet
Price 25036 INR / Piece
Minimum Order Quantity : 5000 Pieces
Physical Form : Tablets
Drug Type : Other, Prescription only
Recommended For : Bacterial infections
Storage Instructions : Store in a cool, dry place below 25C, protect from light
Nanorab-LSR
Price 25620 INR / Piece
Minimum Order Quantity : 5000 Pieces
Physical Form : Tablets
Drug Type : General Medicines
Recommended For : Acidity, Gastroesophageal reflux disease (GERD)
Storage Instructions : Store in a cool and dry place, protected from light
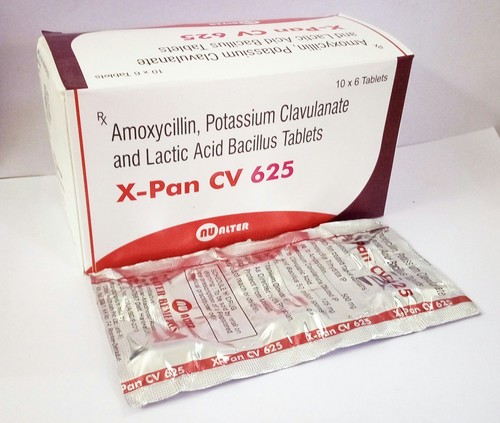
Amoxycillin, Potassium Clavulanate and Lactic Acidm Bacillus Tablets
Price 25045 INR / Piece
Minimum Order Quantity : 5000 Pieces
Physical Form : Tablets
Drug Type : General Medicines
Recommended For : Bacterial infections
Storage Instructions : Store in a cool, dry place away from direct sunlight
Mfr Unit:
Phone: +91-172-4010228
Mobile No.: 9316338826, 08699038826